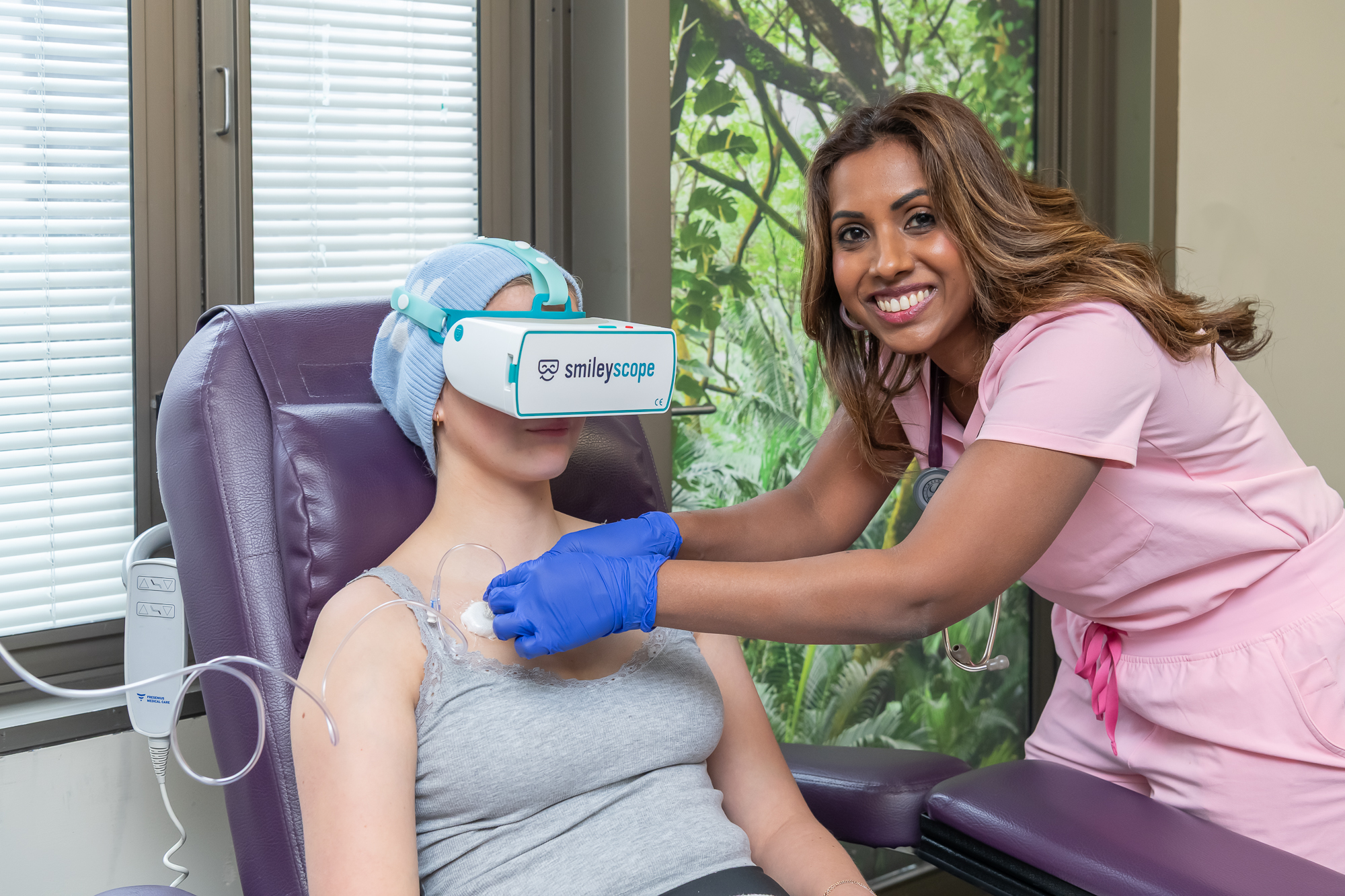
Giving every clinician the power of calm
Smileyscope helps clinicians deliver superior outcomes. Clinically proven to reduce pain by up to 60%, anxiety by up to 40%. Avoid sedation in up to 92%. Trusted across 400,000+ procedures.
Trust by leading healthcare organisations

.svg)







.svg)






Smileyscope delivers effective, drug-free therapies that transform how healthcare teams manage procedural pain and anxiety.
Reduce pain by up to 60%[1]
Reduce anxiety by up to 40%[1]
Avoid sedation in up to 92% of patients[2]
95% of clinicians say procedures are easier[3]
[1] Chan E et al, J Peds, 2019, [2] Johnson B et al, Hosp Peds 2025, [3] Smileyscope Data on File RWEADS-2025v9, 2025

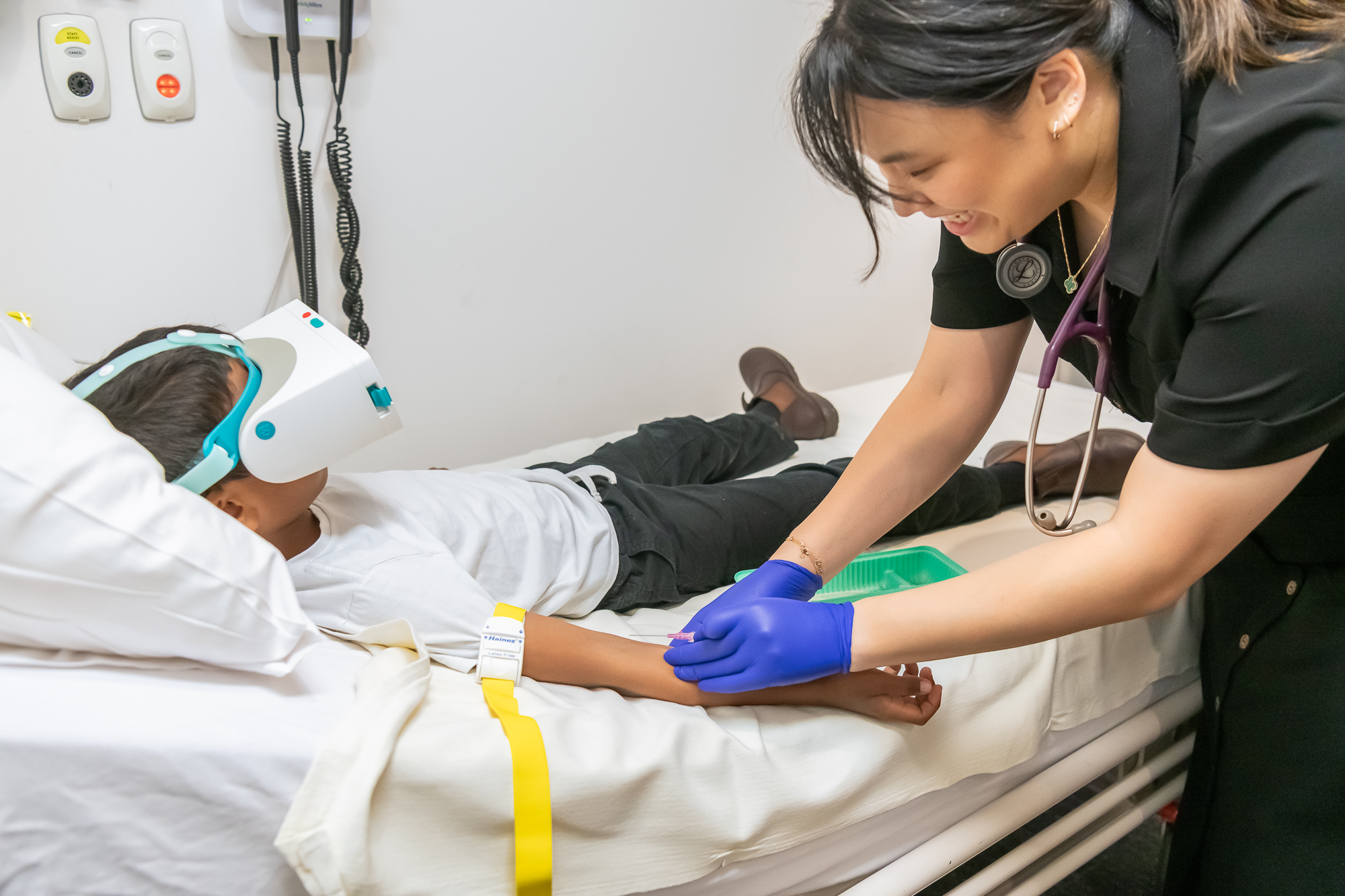
How it works
Step One
Select from touchscreen, introduce and fit it on the patient
Step Two
Perform procedure with our unique Procedural Choreography™, reframing real world sensations into supportive and engaging sensations in virtual reality
Step Three
After the procedure, remove and wipe down the headset - proven to reduce pain by up to 60% and anxiety by up to 40%
Step One
Select from touchscreen, introduce and fit it on the patient
Step Two
Perform procedure, with carefully crafted supportive meditations, alleviating physical and emotional discomfort and support healing
Step Three
After the procedure, remove and wipe down the headset - proven to reduce anxiety in 89% of women
Step One
Select from touchscreen, introduce and fit it on the patient as pre-procedure preparation
Step Two
Smileyscope simulates the MRI environment with graded exposure and gamifies staying still, giving clinicians the ability to assess whether patients can undergo awake MRI
Step Three
Remove the headset and wipe down - the patient is now prepared for their awake MRI, avoiding sedation in 92% of cases
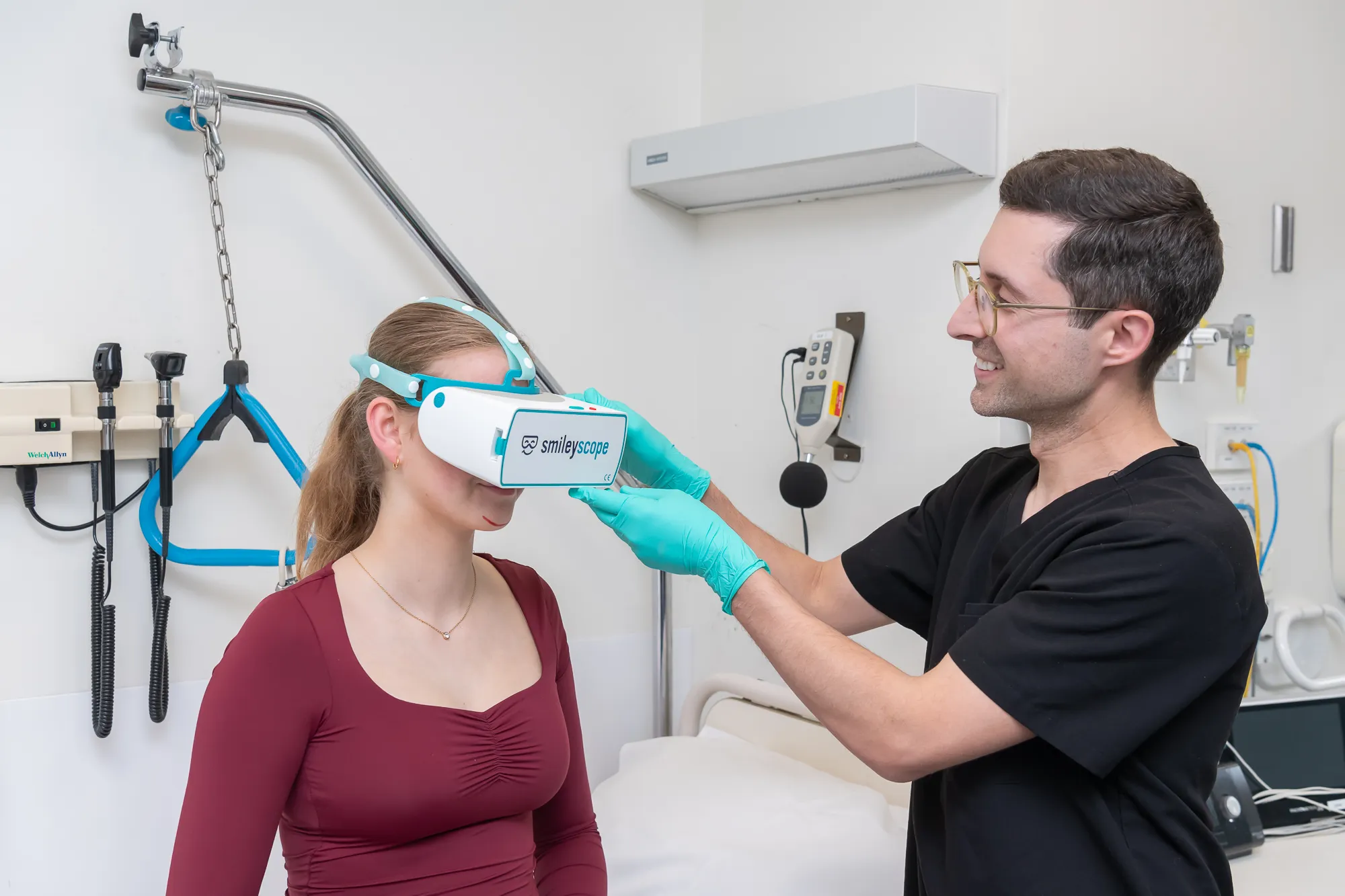
Evidence-based pain and anxiety digital therapies
Transform patient outcomes with digital therapeutics backed by over 20 studies including the largest clinical trial in procedural VR care.
Smileyscope delivers up to 60% pain reduction, up to 40% anxiety reduction, and can avoid sedation up to 92% of the time.
Trusted by leading hospitals worldwide, with FDA, MHRA, TGA, and CE regulatory clearances, supporting over 400,000+ successful procedures and proven clinical outcomes.
Bringing back calm
Transform patient outcomes with digital therapeutics backed by over 20 studies including the largest clinical trial in procedural VR care.

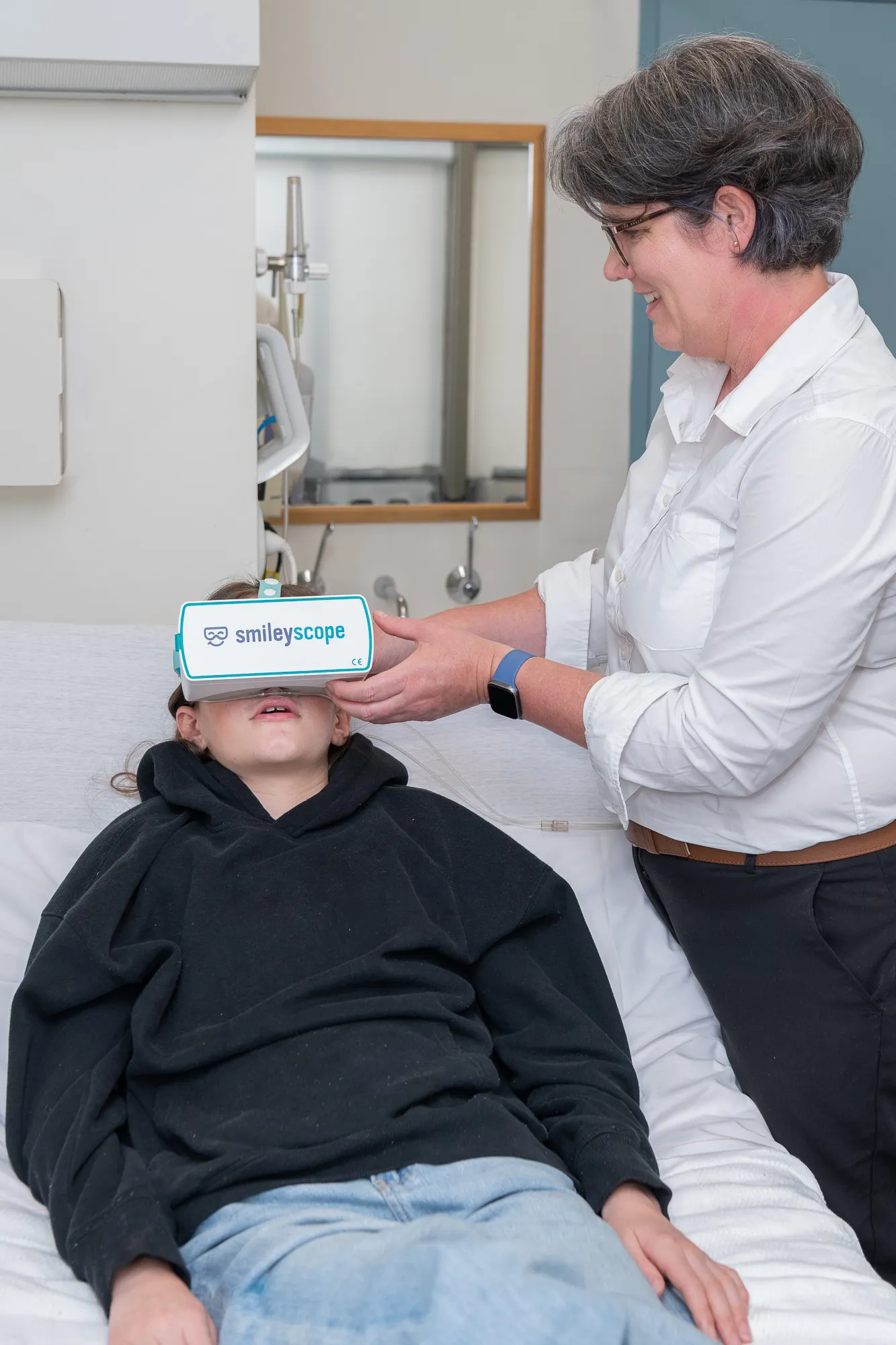
Save valuable clinical time whilst delivering superior patient care
Healthcare teams save time previously spent managing anxious patients, calling additional staff, or administering sedation.
Easy integration: technology that works reliably anywhere, lightning fast to start, no WiFi, does not store patient data.
With procedures running on time, and reduced sedation costs, you'll increase patient throughput whilst dramatically improving the quality of care and satisfaction.
See why 10,000 other clinicians use Smileyscope
Join leading hospitals and clinics delivering superior patient outcomes with evidence-based VR technology. Book a demo to see how Smileyscope transforms procedures in your healthcare setting.